HiFocus Electrode Family
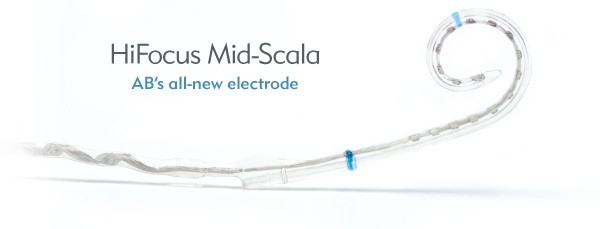
HiFocus Mid-Scala Electrode
Designed for
optimal cochlear placement
for full-spectrum hearing
Unique mid-scala placement
Smallest pre-curved electrode
designed to fit safely
in each individual cochlea
Features Chart
| Unique Features | Why this Matters to You | HiFocus Mid-Scala Electrode |
|---|---|---|
| Designed for Mid-Scala Placement |
The patented design offers lowest insertion forces and no contact with the delicate structures of the cochlea |  |
| Designed for Optimal Insertion | Ideal insertion depth and placement for maximum performance1-3 |  |
| Designed as the Smallest Pre-Curved Electrode |
Easy and optimal surgical placement, designed to fit safely in each individual's cochlea |  |
| Designed for Focused Stimulation |
Patented electrode contacts with Mid-Scala placement provide the perfect platform for current steering |  |
| Designed for the Surgeon | Developed for latest soft surgery techniques, including round window insertion, with dedicated tooling for precise control |  |
| Designed for Reliability | Robust mechanical improvements of the HiRes 90K implant that leads the industry with a 99.8% one-year cumulative survival rate (CSR)4 |  |
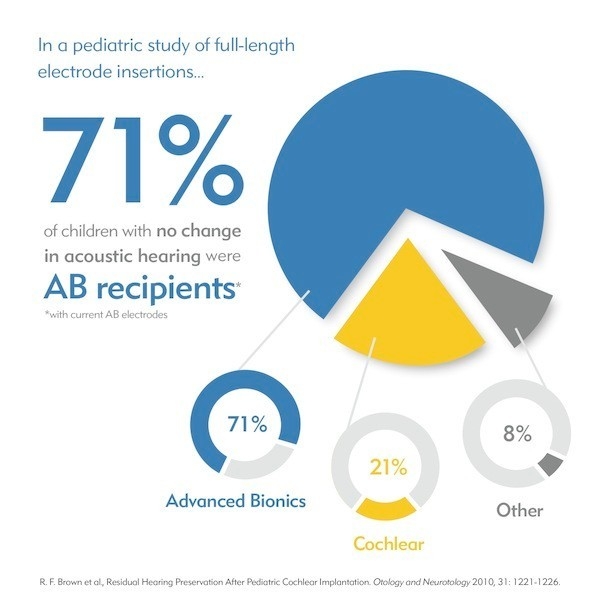
The HiFocus Mid-Scala electrode for HiRes 90K Advantage implants from Advanced Bionics is the industry’s latest innovation in electrode design. Developed through extensive research and using state-of-the-art manufacturing processes, the HiFocus Mid-Scala electrode has been designed for optimal placement in the cochlea.
Featuring the industry’s smallest pre-curved array, the HiFocus Mid-Scala is also the industry’s only pre-curved electrode developed for the latest soft surgery approaches, including round window insertion, to suit surgeon preferences and individual recipient needs. Only AB offers surgeons the choice of using a freehand technique or a special insertion tool uniquely designed for easy control and placement. AB also exclusively provides the ability to reload the electrode in the OR.
HiFocus™ Electrode Family: More Options for Better Hearing
HiFocus™ Mid-Scala Electrode
Offering surgeons precise control of the angle and speed of insertion, the HiFocus Mid-Scala electrode has been developed to insert easily and gently for maximum protection of the cochlea.
HiFocus™ 1j Electrode
AB’s HiFocus 1j electrode is designed for highly effective placement inside the cochlea. With a robust design and ease of implantation, the HiFocus 1j is the electrode of choice for many surgeons worldwide.
HiFocus Helix™ Electrode
AB’s HiFocus Helix electrode is designed to conform to the cochlea’s natural contour. It can be implanted using more than one surgical approach and offers the ability to reload the electrode providing peace of mind to the surgeon.
Most Like Normal Hearing
Every electrode array in the HiFocus family delivers focused stimulation through current steering technology — available only from Advanced Bionics — for hearing that more closely resembles normal hearing. Current steering utilizes 16 independent current sources to increase the number of distinct pitches heard. AB’s patented HiFocus electrode contacts are the ideal platform to deliver AB’s superior sound processing strategies for the best possible hearing outcome.
References
Frijns JHM, Kalkman RK, Vanpoucke FJ, Bongers JS, Briaire JJ. Simultaneous and non-simultaneous dual electrode stimulation in cochlear implants: evidence for two neural response modalities. Acta Otolaryngol. 2009 Apr; 129(4):433-9.
Stakhovskaya O, Sridhar D, Bonham BH, et al. Frequency map for the human cochlear spiral ganglion: Implications for cochlear implants. J Assoc Res Otolaryngol. 2007 8: 220-233.
Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, Gotter BD, Vanderhoof SS, Mispagel K, Heyebrand G, Skinner MW. Factors affecting open-set word recognition in adults with cochlear implants. Ear and Hearing. 2013 Jan 23; Epub.
Carlson ML, Driscoll CL, Gifford RH, Service GJ,Tombers NM, Hughes-Borst BJ, Neff BA, Beatty CW. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol. 2011 Aug;32(6):962-8.
Fraysse B, Macías AR, Sterkers O, Burdo S, Ramsden R, Deguine O, Klenzner T, Lenarz T, Rodriguez MM, Von Wallenberg E, James C. Residual hearing conservation and electroacoustic stimulation with the nucleus 24 contour advance cochlear implant. Otol Neurotol. 2006 Aug 27 (5):624-33.
Advanced Bionics Reliability Update. 2012.