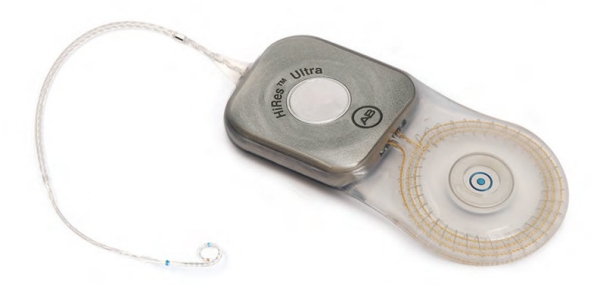
High Resolution – Low Profile
HiRes Ultra Cochlear Implant

The HiRes™ Ultra cochlear implant features the thinnest implant profile from AB and is built on the proven HiRes electronics technology. The discreet package requires minimal drilling, making it suitable for all implant recipients — adults and children — and exceeds industry standard for impact resistance.1
The HiRes Ultra features the HiFocus™ Mid-Scala electrode, designed to protect the delicate structures of the cochlea and to suit individual patient anatomy and surgical preferences for the best possible hearing outcomes. Choosing AB allows you or your child to benefit from tomorrow’s technology with today’s implant.
High Resolution
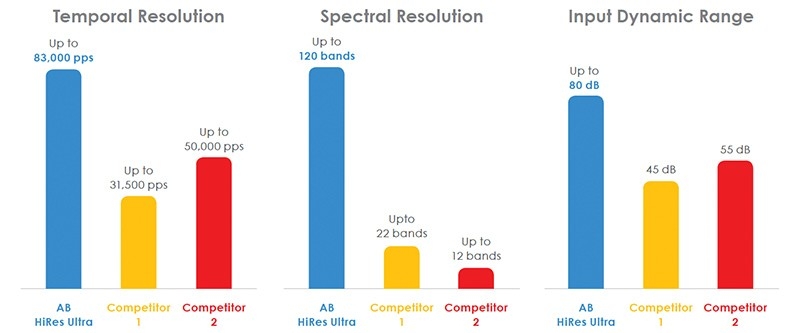
- The only device with 16 independent current sources that facilitate current steering.
- Ability to stimulate 120 distinct spectral bands leading to unsurpassed temporal resolution and better pitch differentiation.2
- The fastest stimulation rate combined with the widest input dynamic range allows for the most natural hearing possible.3,4,5
- Circuitry provides nearly unlimited ways to deliver stimulation – for future technology not invented yet.
Low Profile
- Minimal drilling required, with a shallow 1mm ramped recess.
- The thin 4.5mm profile and small footprint make it suitable for all implant recipients — adults and very young children.

MRI Compatible
- FDA and TÜV approved for 1.5T with the magnet in place – an MRI Antenna Coil Cover and a simple head-bandage procedure is all that is required, no surgical procedure is necessary
- FDA and TÜV approved for 3T MRI with the magnet removed – the magnet can be taken out easily and replaced through a small incision, without the inconvenience of removing the implant
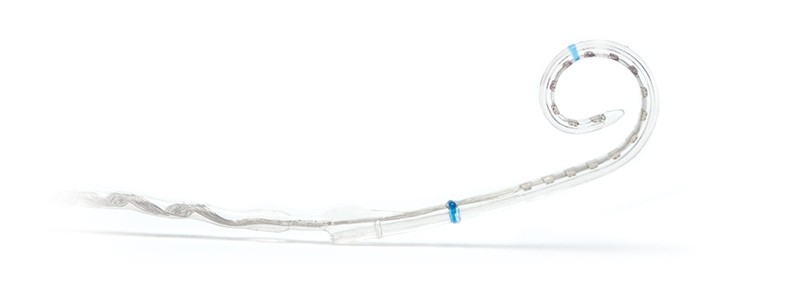
HiFocus Mid-Scala Electrode
The HiFocus Mid-Scala electrode has been designed for ideal positioning in the Scala Tympani and full spectrum coverage for maximum performance6, while protecting the delicate cochlear structure.
Single Sided Deafness
The HiRes Ultra cochlear implant and HiFocus Mid-Scala electrode have been approved by TÜV, a European Notified Body, to be implanted in patients with unilateral (asymmetric)hearing loss, also known as single-sided deafness (SSD).
Current steering is the process of using multiple current sources to stimulate two electrodes at the same time so that recipients hear additional pitches compared to when either electrode is stimulated alone. The industry’s innovation leader, AB offers HiRes Fidelity 120™*, a breakthrough in sound processing technology that uses current steering to increase the number of pitches heard. Studies have shown that compared to using other sound processing strategies, AB recipients using HiRes Fidelity 120 hear speech better in noise and experience improvements in music and sound quality.7,8,9 HiRes™ Optima sound processing provides the same rich and detailed sound with an improved battery life.
* Not approved for pediatric use in the U.S.
Mechanical improvements of the HiRes 90K™ implant that leads the industry with a 99.84% one-year cumulative survival rate (CSR),10 a measure that defines the likelihood of a device continuing to function over time. You can rest assured that an AB cochlear implant will continue to work so that you may always hear your best.
Since our inception in 1993, AB has consistently made the industry’s leading technological hearing advancements, including superior temporal resolution, spectral resolution, dynamic range, and stimulation flexibility designed to be the most like normal hearing.
References
EN 45502-2-3:2010. Active Implantable Medical Devices. Particular Requirements for Cochlear and Auditory Brainstem Implant Systems.
Firszt JB, Holden L, Reeder R, Skinner MW. 2009. Spectral Channels and Speech Recognition in Cochlear Implant Recipients using HiRes 120 Sound Processing. Otology and Neurotology 30:146-152.
Koch DB, Osberger MJ, Segel P, Kessler DK. (2004) HiResolution and conventional sound processing in the HiResolution Bionic Ear: using appropriate outcome measures to assess speech-recognition ability. Audiology and Neurotology, 9:214-223.
Spahr A, Dorman MF, Loiselle LH. 2007. Performance of Patients Using Different Cochlear Implant Systems: Effects of Input Dynamic Range. Ear and Hearing. 28:260-275.
Firszt JB, Holden L, Reeder R, Skinner MW. (2009) Speech recognition in cochlear implant recipients: comparison of standard HiRes and HiRes 120 sound processing. Otology and Neurotology 30:146–152.
Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, Gotter BD, Vanderhoof SS, Mispagel K, Heyebrand G, Skinner MW. Factors affecting open-set word recognition in adults with cochlear implants. Ear and Hearing. 2013 Jan 23; Epub.
Firszt JB, Holden L, Reeder R, Skinner MW. 2009. Spectral Channels and Speech Recognition in Cochlear Implant Recipients using HiRes 120 Sound Processing. Otology and Neurotology 30:146-152.
Brendel M, Buchner A, Kruger B, Frohne-Buchner C, Lenarz T. 2008. Evaluation of the Harmony Sound Processor in Combination with the Speech Coding Strategy HiRes 120. Otol Neurotology 29:199-202.
Oleson J, Lesh S, Gfeller K, Knutson J. The Effect of Advanced Bionics’ HiRes 120 on Self-Report of Music Enjoyment. Poster Presentation at the 10th International Conference on Cochlear Implants and Other Implantable Auditory Technologies, April 10-12, 2008, San Diego, CA.
Advanced Bionics 2016 Mid-Year Cochlear Implant Reliability Report.